Digitalisierung der Supply Chain, Anpassung Ihres Unternehmens an neue Entwicklungen
Wir helfen Life-Science-Unternehmen bei Planung, Management und bei Lieferung ihrer Verpackungen, Marketingkommunikationsmaterialien und digitalen Assets sowie bei der Optimierung ihrer digitalen Supply Chain.
-

Packaging Artwork & Labelling Solutions
Write your caption hereTaste -

Strategic Consultancy
Our consulting team provides strategic advice on supply chain,
packaging/labelling, regulatory, quality, and marketing communications.
Taste -

Creative & Digital
Our team delivers best-in-class creativity for the Life Science industry.
Helping you to visually communicate effectively through all channels.
Taste -
Strategic Outsourcing - BPS
With a highly competitive and regulated marketplace, it is becoming
the norm within the Life Sciences industry to outsource specialised tasks.
Taste -

Managed Services
We manage complex supplier relationships taking full end-to-end
responsibility for the just-in-time delivery that help bring your products to market.
Taste -
Software Solutions
Our purpose-built platform, GLAMS, is the world’s only workflow
management system designed specifically for the Life Science industry.
Taste
Folientitel
Write your caption hereTaste
Folientitel
Write your caption hereTaste
Folientitel
Write your caption hereTaste
Folientitel
Write your caption hereTaste
Folientitel
Write your caption hereTaste
Folientitel
Write your caption hereTaste
Wir unterstützen Life Science-Unternehmen bei der Konzeption, Verwaltung und Lieferung von Verpackungsgrafiken, Marken und digitalen Assets
-

Dienstleistungen für die Verpackung von Kunstwerken und Etiketten
We provide global leadership and best-in-class customer engagement
in the provision of packaging artwork and labelling services for the Life Science industry.
Weiterlesen -

Strategische Beratung
Our consulting team provides strategic advice on supply chain,
packaging/labelling, regulatory, quality, and marketing communications.
Taste -

Kreative und digitale Dienstleistungen
Our team delivers best-in-class creativity for the Life Science industry.
Helping you to visually communicate effectively through all channels.
Taste -

Strategisches Outsourcing (BPS)
With a highly competitive and regulated marketplace, it is becoming
the norm within the Life Sciences industry to outsource specialised tasks.
Taste -

Managed Services
We manage complex supplier relationships taking full end-to-end
responsibility for the just-in-time delivery that help bring your products to market.
Taste -

Softwarelösungen
Our purpose-built platform, GLAMS, is the world’s only workflow
management system designed specifically for the Life Science industry.
Taste
Sechs Lösungen, ein Team und eine digitalisierte Supply Chain
Qualität garantiert
Mit unseren Lösungen können Life-Science-Unternehmen ihre Supply Chain digitalisieren und integrieren
„Aussagekräftige Daten in Echtzeit zu erfassen und auszutauschen, bringt Unternehmen und Kunden enger zusammen.“
Garantierte Qualität
Über uns
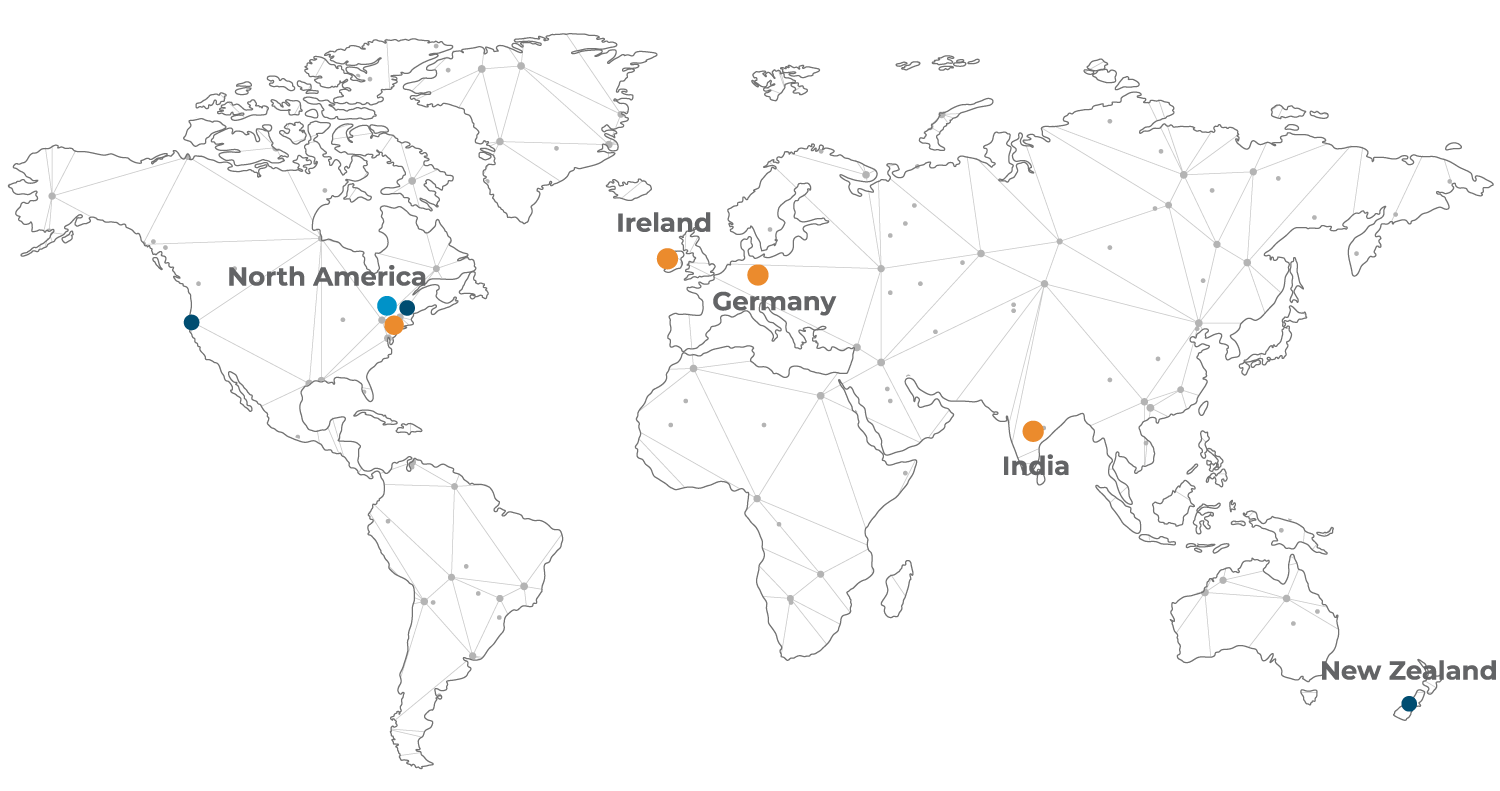
Global Centres of Creative and Packaging Excellence
+40
+350
100
7%
Neuesten Nachrichten

Neuesten Nachrichten