Artwork Management
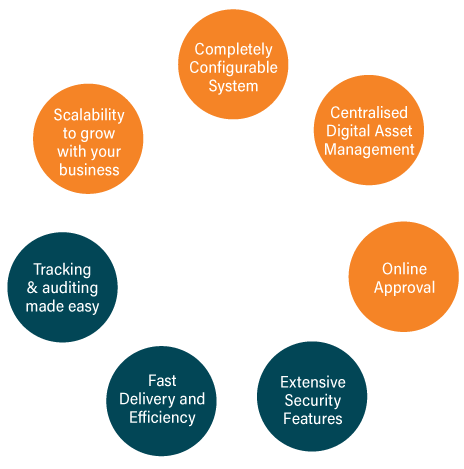
Introducing GLAMS 6.0, the latest version of Perigord’s digital enterprise and configurable system. The GLAMS AMS product suite delivers global advantage and best-in-class regulatory compliance, providing validated packaging supply chain workflow management solutions to the Life Science industry.
Scalable GLAMS AMS Solutions for
Life Science Companies
With a range of scalable solutions supporting emerging to large global Life Science companies, our GLAMS AMS solutions are designed to help organisations manage the complex workflows surrounding artwork and labelling.
To find out which GLAMS AMS solution best fits your organisation:

GLAMS – Built Exclusively for the Pharmaceutical Industry
From launching your first product to achieving global dominance, GLAMS can help you.
GLAMS
artwork management software has been designed specifically to meet the unique regulatory requirements of pharmaceutical, medical device, and biotech industries.
Focusing on artwork management, we created GLAMS AMS, to not only control all the workflows and processes that surround the creation, change management, and regulatory approval processes required in the generation of global packaging artwork and labelling assets when bringing a product to market, but also to streamline the entire process.
When selecting GLAMS AMS as your artwork management software and content approval system, you are selecting a platform that is:
- user-friendly
- available with a full validation pack
- fully regulatory compliant, supporting your audit requirements
- proven to reduce artwork revision cycle times by up to 70%
- a driver of local and global collaboration
GLAMS AMS is now deployed in over 60 global Life Science organisations and is used to manage thousands of artwork change requests daily. GLAMS AMS is continuously evolving to meet the ever-changing needs of the Life Science industry and the increasing demands of global regulation.

Benefiting All Functions and Stakeholders Across the Supply Chain, Locally and Globally
Packaging labelling and artwork management involves collaboration with internal and external stakeholders. Click below to see how GLAMS AMS can benefit you:

QUALITY
- We act as a Pharma company with a large global Quality Assurance team
- We are experts in artwork & labelling Pharma processes
- Easy to Audit – GLAMS tracks every job from beginning to end.
- GLAMS is built for the highly regulated pharma industry to cGMP standards
- Full Transparency – Get in-depth, live data on KPIs, Lead Time Reports, Rejection Reasons Reports and much more.
SUPPLY CHAIN MANAGEMENT
- Identify bottlenecks and delays quickly
- Spot problems easily across local & global locations
- Business Intelligence Reports will clearly highlight any issues
- Full knowledge of issues means it is easy to improve your processes
PROCUREMENT
- Rapid deployment – dedicated expert implementation team
- Expert Team – Project Manager, Business Analyst, Validation Engineer & Key Account Manager
- Validation pack provided by Perigord – no need to build your own pack
- Pharmaceutical labelling & artwork industry experts who understand the challenges you face
- Integrates seamlessly
ARTWORK STUDIOS
- Easy graphics + text verification with built-in media proofing tools
- Comment on tasks – no more email chains.
- Dashboard overview of all tasks assigned to you and your team
- Notifications for due tasks, approvals and more
PRINT SUPPLIERS
- Always work with the latest master files
- Secure easy file transfer – no more emails, no more incompatible formats
- Easily recommend changes while staying inside the audit trail
- Notification of new artwork immediately once it enters the system
PROJECT MANAGERS
- Control overall projects and stakeholders
- Clean up approval bottlenecks and reduce costs
- Process control with automated pharma workflows
- Improve KPI’s, see significant improvements in cycle time and RFT.
REGULATORY
- No more audit headaches – Full traceability with a full audit trail of all labelling & artwork packaging
- Compliant – European Annex 11 and U.S Title 21 CFR Part 11 guidelines
- Electronic signatures support to speed up the approval process
- Built exclusively for the heavily regulated pharmaceutical industry
Discover more by
Downloading the Analytics White Paper
Main reasons why regulated Life Science companies buy GLAMS
Compliance
GLAMS is compliant with GMP, EU Annex 11, and 21 CFR PART 11 standards, ensuring adherence to global regulatory requirements (FDA, Canada Health & EMA, etc.). We've designed GLAMS according to GAMP5 guidelines, providing a validation pack for seamless system implementation.
Standard
Working Processes
Our Pharma Experts at GLAMS provide guidance on artwork processes, offering both predefined industry workflows or seamless integration with your existing processes. Our implementation team maps and integrates your current processes, addressing any additional needs.
Seamless
Tracking
GLAMS ensures flawless artwork approval, tracking every step. Instantly generate audit reports, view project overviews, and identify bottlenecks. Enhanced analytics offer deep insights into performance metrics.
Scaling and
Integration
GLAMS grows with your business, offering modular expansion for functionality, storage, users, and workflows. It seamlessly integrates with systems like SAP or Oracle, providing ongoing management and support for a smooth growth journey.
Advanced Artwork Labelling Production Analytics
All this information flows into easy to read, exportable reports, that offers a live view of your team's performance. GLAMS Analytics can expand this even further, by giving you in-depth Business Intelligence Reports capabilities for deeper dives into big data-based metrics.